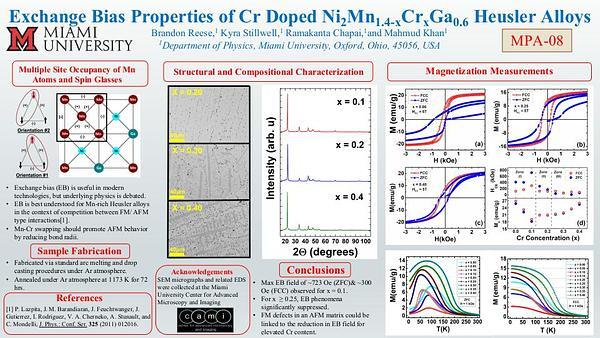
Would you like to see your presentation here, made available to a global audience of researchers?
Add your own presentation or have us affordably record your next conference.
Co3O4 is a transition metal oxide with atypical exchange interactions that propagate through nonmagnetic ions giving rise to weak antiferromagnetism1. Thus, small
changes to the exchange pathways can lead to large changes in the observed magnetism. Cu, Ni, and Fe-doped Co3O4 nanoplates were synthesized (Fig. 1) to investigate the differences in
magnetic response as a result of the new exchange interactions, including contributions from the surface and core2. Through the addition of other transition metal ions we disrupt the
intrinsic exchange interactions and investigate the effect on the electronic and magnetic structures. The electronic structure of doped materials can be altered to form ligand holes in the
oxygen band. Instead of the holes residing on the metal ion – creating a mixed valence structure of 2+ and 3+, the holes occupy the oxygen band creating a ligand hole3,4. This manifests
in interesting ways such as modifying the exchange interactions through delocalization of the electrons.
The addition of dopants into the A (tetrahedral) site leads to a change in the number of unpaired spins, decreasing the strength of the extended exchange interactions responsible for the
antiferromagnetic order. Occupation of the B (octahedral) site leads to stronger ferromagnetic interactions. By replacing the Co3+ ions which have no magnetic moment with Cu, Ni, or Fe
ions, we introduce unpaired spins into the B site. Fe-doped Co3O4 shows the strongest exchange interactions. For example, 25% atm. Fe shows the largest magnetic saturation at roomtemperature
compared to similar dopings of Ni, and Cu (Fig. 2). The strong magnetic response is a result of Fe3+ occupying the B site interacting with Co2+ on the A site. Interestingly,
both Cu and Ni occupy the B site with a 2+ oxidation state (replacing Co3+) which disrupts the charge balance of the system. This forces Cu and Ni to appropriate electrons from the
surrounding oxygen ions. This behaviour can modify the existing exchange interactions, similar to what happens in the high temperature superconducting cuprates.
References
1 - W.L. Roth, J Phys. Chem. Solids, Vol. 25, 1, p.1-10 (1964).
2 - M. Shepit, V.K. Paidi, C.A. Roberts, et al., Sci Rep., Vol. 10, 20990 (2020).
3 - M. Shepit, V.K. Paidi, C.A. Roberts, et al., Phys. Rev. B, Vol. 103, 024448 (2021).
4 - G. Sawatsky, R. Green, Quantum Materials: Experiment and Theory, Autumn School on Correlated Electrons (Jülich), (2016).